Introduction.Hematopoietic stem cells (HSCs) are responsible for maintaining the homeostasis of the blood and immune systems. This process must rapidly adapt to bleeding, infection, injury, and disease. HSCs reside in specialized microenvironments in the bone marrow, called “niche”. Several bone marrow cell types have been proposed as the key components of the niche. It is well established that HSCs rely on molecular signals from the niche cells to survive, migrate, proliferate, and differentiate. Recent studies suggest that HSCs differentiate heterogeneously. Additionally, the bone marrow is a heterogeneous environment consisting of more than thirty cell types. This study will test the hypothesis that individual HSCs are engaged in heterogeneous intercellular signaling in the bone marrow.
Materials and Methods.We utilized CellChat (Jin et al., 2021), a database for ligand-receptor pairs and associated pathways, to predict cell-cell signaling at the single cell level. We used single cell RNA sequencing data from mouse hematopoietic stem and progenitor cells, blood and immune cells, and non-hematopoietic cells from the bone marrow to determine putative cell-cell interactions. The predicted signaling interactions were then adjusted based on the cell-cell spatial interaction revealed by PhenoCycler measurement (Akoya Biosciences). Mouse bone marrow transplantation experiments were performed to test the functional significance of the identified signaling pathway interactions.
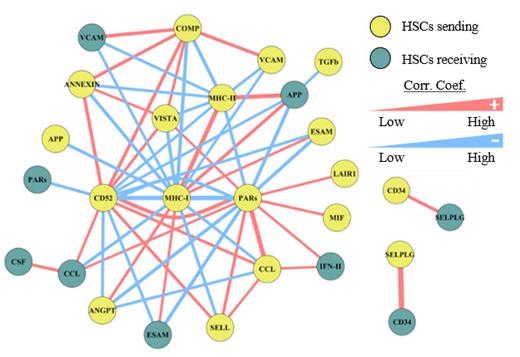
Results.We found that individual HSCs send and receive different levels of molecular signals across various signaling pathways. In addition to the expected interactions with the known HSC niche cells, we also found new interactions between HSCs and their progeny cells including myeloid progenitors and immune cells. These cell-cell signaling interactions are consistent with the spatial interaction frequencies of the corresponding cell types as revealed by our imaging data from the PhenoCycler analysis. Further, we found that some signaling pathways are positively or negatively correlated across individual HSCs, indicating complex intercellular signaling networks. The most significant negative correlation was found between HSCs sending MHC-I signals and sending PARs signals (Figure). Moreover, this MHC-I/PARs signaling correlation network also involves signaling from the HSC niche, including those that are known to play roles in HSC homing such as ESAM and VCAM. Therefore, we tested the engraftment efficiency of HSCs that receive PARs and those that do not using the HSC mouse transplantation model. We found that PAR1 + HSCs engraft much less efficiently. Finally, we present the HSC Interaction Data Explorer (HIDE), a database that comprehensively depicts the interactions between HSCs and other bone marrow cells including the involved signaling pathways, ligands and receptors of the pathways, and the pathway correlations.
Conclusion.Our study uncovered novel interactions of HSCs with myeloid progenitor cells and immune cells, which were validated by spatial interaction data. These interactions indicate feedback signaling in regulating blood cell regeneration. Our findings further revealed signaling pathway correlation networks across individual HSCs, and we showed their functional significance using transplantation experiments. Finally, we present HIDE as a resource that informs the interactions between HSCs and various bone marrow cells, the relevant signaling pathways, and the pathway correlations.
Figure Legend. HSC signaling correlation networks. Each node shows a signaling pathway and direction (e.g., HSCs sending MHC-I). The edges represent the spearman correlation score.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal